Specifications
- 1.8mm x 130cm tracking catheter
-
1.8mm x 130cm tracking catheter Part Number - P18130K
Introducer size - 5F (greater than 1.8 mm)
Working length - 130 cm
Guidewire diameter - 0.014”
-
- 1.8mm x 149cm tracking catheter
-
1.8mm x 149cm tracking catheter Part Number - P18149K
Introducer size - 5F (greater than 1.8 mm)
Working length - 149 cm
Guidewire diameter - 0.014”
-
- 2.2mm x 130cm tracking catheter
-
2.2mm x 130cm tracking catheter Part Number - P22130K
Introducer size - 6F (greater than 2.2 mm)
Working length - 130 cm
Guidewire diameter - 0.014”
-
- 2.2mm x 149cm tracking catheter
-
2.2mm x 149cm tracking catheter Part Number - P22149K
Introducer size - 6F (greater than 2.2 mm)
Working length - 149 cm
Guidewire diameter - 0.014”
-
- 2.4mm x 127cm deflecting catheter
-
2.4mm x 127cm deflecting catheter Part Number - PD24127K
Introducer size - 7F (greater than 2.4 mm)
Working length - 127 cm
Guidewire diameter - 0.014”
-
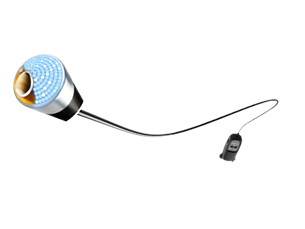
- Phoenix guidewire
-
Phoenix guidewire Part Number - PG14300LF
Product - Phoenix guidewire
Length - 300 cm
Wire Support Level - Light
-
- 1.5mm x 149cm tracking catheter
-
1.5mm x 149cm tracking catheter Part Number - P15149K
Working length - 149 cm
Introducer size - 4F (greater than 1.5mm)
Guidewire diameter - 0.014“
-
- 2.2mm x 130cm pre-deflected catheter
-
2.2mm x 130cm pre-deflected catheter Part Number - PD22130K
Working length - 130 cm
Introducer size - 6F (greater than 2.2 mm)
Guidewire diameter - 0.014”
-
- 2.4mm x 130cm pre-deflected catheter
-
2.4mm x 130cm pre-deflected catheter Part Number - PD24130K
Working length - 130 cm
Introducer size - 7F (greater than 2.4 mm)
Guidewire diameter - 0.014”
-
- 1.8mm x 130cm tracking catheter
-
1.8mm x 130cm tracking catheter Part Number - P18130K
Introducer size - 5F (greater than 1.8 mm)
-
- 1.8mm x 149cm tracking catheter
-
1.8mm x 149cm tracking catheter Part Number - P18149K
Introducer size - 5F (greater than 1.8 mm)
-
- 1.8mm x 130cm tracking catheter
-
1.8mm x 130cm tracking catheter Part Number - P18130K
Introducer size - 5F (greater than 1.8 mm)
Working length - 130 cm
Guidewire diameter - 0.014”
-
- 1.8mm x 149cm tracking catheter
-
1.8mm x 149cm tracking catheter Part Number - P18149K
Introducer size - 5F (greater than 1.8 mm)
Working length - 149 cm
Guidewire diameter - 0.014”
-
- 2.2mm x 130cm tracking catheter
-
2.2mm x 130cm tracking catheter Part Number - P22130K
Introducer size - 6F (greater than 2.2 mm)
Working length - 130 cm
Guidewire diameter - 0.014”
-
- 2.2mm x 149cm tracking catheter
-
2.2mm x 149cm tracking catheter Part Number - P22149K
Introducer size - 6F (greater than 2.2 mm)
Working length - 149 cm
Guidewire diameter - 0.014”
-
- 2.4mm x 127cm deflecting catheter
-
2.4mm x 127cm deflecting catheter Part Number - PD24127K
Introducer size - 7F (greater than 2.4 mm)
Working length - 127 cm
Guidewire diameter - 0.014”
-
- Phoenix guidewire
-
Phoenix guidewire Part Number - PG14300LF
Product - Phoenix guidewire
Length - 300 cm
Wire Support Level - Light
-
- 1.5mm x 149cm tracking catheter
-
1.5mm x 149cm tracking catheter Part Number - P15149K
Working length - 149 cm
Introducer size - 4F (greater than 1.5mm)
Guidewire diameter - 0.014“
-
- 2.2mm x 130cm pre-deflected catheter
-
2.2mm x 130cm pre-deflected catheter Part Number - PD22130K
Working length - 130 cm
Introducer size - 6F (greater than 2.2 mm)
Guidewire diameter - 0.014”
-
- 2.4mm x 130cm pre-deflected catheter
-
2.4mm x 130cm pre-deflected catheter Part Number - PD24130K
Working length - 130 cm
Introducer size - 7F (greater than 2.4 mm)
Guidewire diameter - 0.014”
-
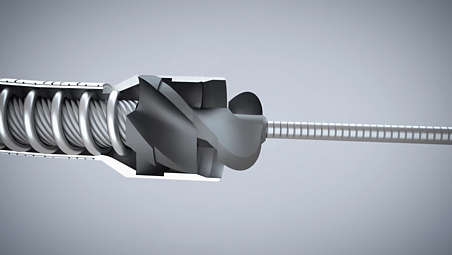
- 1. Rotational atherectomy refers to the Phoenix family of products. 2.4mm deflecting catheter and 2.2 mm auto-deflected catheter have directional cutting ability.
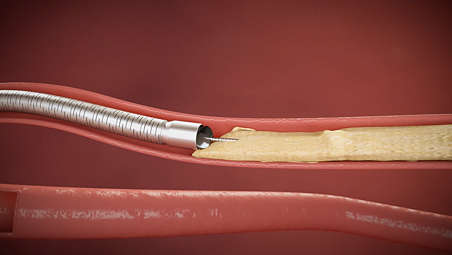
- 2. Monitor flow of excised material into the disposal reservoir during operation of the Catheter. If flow of excised material ceases during the procedure, this is a sign that the Catheter drive system (cutter, torque shaft, or Handle motor drive) may not be operating properly.
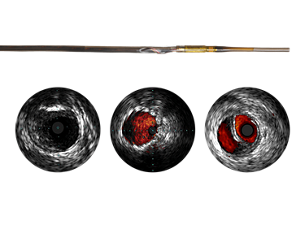
- 3. The Phoenix atherectomy 1.5 mm tracking catheter is indicated for vessels of 2.0 mm in diameter or above, the 1.8 mm tracking catheter is indicated for vessels 2.5 mm in diameter or above. The Phoenix 2.2 mm tracking and deflected as well as the 2.4 mm deflecting catheters are indicated for vessels of 3.0 mm in diameter or above. While the 1.5mm and 1.8 tracking as well as the 2.2 mm tracking and deflecting catheters are indicated for femoral, popliteal, or distal arteries located below the knee, the Phoenix 2.4 mm deflecting catheter is indicated for femoral and popliteal only. Refer to product IFU for detailed instructions. Warning: do not use the Phoenix atherectomy system in vessels smaller than the indicated size or harm to patient (vessel perforation, dissection or injury) could occur.
- 4. Davis T, Ramaiah V, Niazi K, Martin Gissler H, Crabtree T. Safety and effectiveness of the Phoenix Atherectomy System in lower extremity arteries: Early and midterm outcomes from the prospective multicenter EASE study. Vascular. 2017 Dec;25(6):563-575. The Phoenix atherectomy 1.5 mm tracking and 2.2 mm deflecting catheter were not include in the EASE trial.
- 5. Case study results are not predictive. Results in other cases may vary.
- 6. Case performed by Dr. Joseph Griffin at Baton Rouge General Hospital.
- 7. Center mass cutting device when the guidewire is centered.
- 8. Case performed by Dr. William Crowder in Jackson, Mississippi.
- 9. Case performed by Dr. Tom Davis in Detroit, MI.
- Product availability is subject to country regulatory clearance. Please contact your local sales representative to check the availability in your country.
- Phoenix atherectomy system is distributed by InterMed in New Zealand.
- Always read the label and follow the directions for use.
- Philips medical devices should only be used by physicians and teams trained in interventional techniques, including training in the use of this device.
- Philips reserves the right to change product specifications without prior notification.
- ©2025 Koniklijke Philips N.V. All rights reserved. Trademarks are the property of Koninklijke Philips N.V. or their respective owners.